The Basophil Activation Test: An Application of Flow Cytometry for Allergy Testing in Clinical Trials
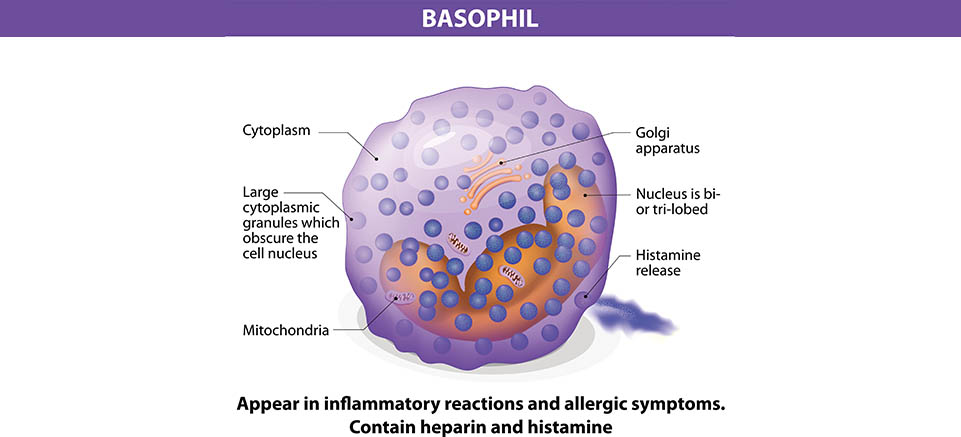
Allergies are one of the fastest growing maladies affecting first world countries. In fact, before 1980 peanut allergies, now so commonly and dangerously found in children, were virtually unheard of in both medical journals and the media. In the USA alone, the CDC reports that between 1997 and 2008, food allergies in children have risen by an astonishing 50 percent (https://www.foodallergy.org/life-with-food-allergies/food-allergy-101/facts-and-statistics). Perhaps most disconcerting of all, the medical community has very few tools with which to study what is causing this sudden rise in allergic reactions, or even to properly diagnose food allergies. Currently, the gold standard test for food allergy diagnosis is the oral food challenge (OFC), where under medical surveillance the patient is subjected to the suspected allergen. OFCs are often, at best, a stressful experience for patients as they are forced to experience the very allergic reaction which they are trying to avoid. Skin prick tests and specific IgE tests are commonly used in combination as an alternative to OFCs, however the rates of both false negatives and false positives for these tests are much higher than is ideal. Furthermore, both OFCs and skin prick tests carry the risk of further sensitizing the patient to their allergen, causing a more drastic reaction than was previously experienced. For years medical researchers have been endeavoring to develop an in vitro test to limit patient sensitization and stress. The Basophil Activation Test (BAT) is the most promising result of this search. The BAT is a highly specific and sensitive diagnostic tool which is fairly simple to perform and, most importantly, does not require the patient to experience any sort of allergic reaction.
Basophils play a pivotal role in IgE mediated allergic reactions. When a molecule binds to IgE, it triggers the exocytosis of granules filled with histamine, proteoglycans, and proteolytic enzymes. Once released, these compounds go on to cause an inflammatory response in surrounding tissues. After exocytosis, cell marker CD63 which was previously located within the cytoplasm is exposed and becomes a part of the outer cell membrane. Cell marker CD203c is also upregulated during cell activation, although by slightly different mechanisms. The BAT works by staining for both CD63 and CD203c and then running the cells through a flow cytometer to detect the presence of activated basophils in blood. The BAT is therefore very phenotypically sensitive. It does not just measure the symptoms of an allergy, but the actual state of the cells causing the allergic reaction. This means that allergies and sensitizations that are either asymptomatic or cause a very small reaction that may be missed by a skin prick test or OFC can be properly diagnosed by the BAT. To test the reliability and efficacy of the BAT at FCSL, we performed a short exploratory study using a commercially available basophil activation kit. Blood was drawn and then incubated under 3 separate conditions: stimulated with fMLP(N-Formylmethionine-leucyl-phenylalanine),stimulated with dust mite allergen, and unstimulated. fMLP serves as a positive control because of its ability to cause potent degranulation reactions in basophils. Each sample was stained for CD203c and CD63 during incubation as well. After incubation, the samples were lysed, washed and analyzed using a flow cytometer.

Figure 1 – The data on the left came from a subject (Patient 1) with a previously diagnosed dust mite allergy. Patient 4 had never expressed symptoms to dust allergens and tested negative for dust mite antigen by skin prick testing. As expected, Patient 1’s blood was highly positive for both CD63 and CD203c after being stimulated with dust mite allergen, while patient 4’s blood was not. The positive and negative controls for both patients as behaved as expected showing that the assay was well controlled.
These results are consistent with other research that has been done using BATs. Santos et al. published an article in The Journal of Allergy and Clinical Immunology exploring the BAT’s ability to detect peanut allergy. (Santos, 2014) They found that in a population of 104 peanut allergic, peanut sensitized, and non allergic children, the BAT was able to diagnose allergy with 97% accuracy. Furthermore, two thirds of the children were spared unnecessary OFCs because of the accuracy of the BAT. When the BAT was combined with a skin prick test or serum IgE test, only 3 percent of the children needed to undergo any OFC at all. The BAT has also shown promising results in diagnosing hazelnut (Brandstrom et al., 2015) , egg(Ocmant et al.,2009), cow’s milk (Sato et al.,2010) and wheat allergies (Tokuda et al.,2009). The table below summarizes these studies’ data.
| Food | Population Size | Sensitivity | Specificity |
| Hazelnut | 40 | 100 | 97 |
| Egg | 67 | 77 | 100 |
| Cow’s Milk | 50 | 89 | 83 |
| Wheat | 58 | 85 | 77 |
These results and the results of our testing show the promise of the BAT as a clinical research tool. As the BAT continues to be developed, hopefully it can one day replace the OFC as the gold standard for allergy diagnosis, leading to care that is not only more accurate but also more convenient for both patients and doctors.
FCSL is a contract flow lab that provides high throughput and high capacity flow cytometry services, running multiple flow cytometers with up to 10 color antibody panels daily. We are proficient in processing a multitude of specimen types including whole blood, frozen PBMCs along with cell culture and tissue processing capabilities. Our flexibility in handling so many specimen types allow for the support of a wide range of flow cytometry assays including: immunophenotyping/lymphocyte subset analysis, receptor occupancy, functional assays and cell viability/apoptosis measurements. Our expert staff is always available to help guide you through these tests and we welcome clients to visit our facility. We encourage sponsor engagement throughout the process. Contact us for more information!
References:
Brandstrom J, Nopp A, Johansson SG, Lilja G, Sundqvist AC, Borres MP, Nilsson C. Basophil allergen threshold sensitivity and component resolved diagnostics improve hazelnut allergy diagnosis. Clin Exp Allergy. 2015;45:1412. doi: 10.1111/cea.12515. [PubMed] [Cross Ref]
Ocmant A, Mulier S, Hanssens L, Goldman M, Casimir G, Mascart F, Schandene L. Basophil activation tests for the diagnosis of food allergy in children. Clin Exp Allergy. 2009;39(8):1234–1245. doi: 10.1111/j.1365-2222.2009.03292.x. [PubMed] [Cross Ref]
Santos AF, Douiri A, Becares N, Wu SY, Stephens A, Radulovic S, Chan SM, Fox AT, Du Toit G, Turcanu V, et al. Basophil activation test discriminates between allergy and tolerance in peanut-sensitized children. J Allergy Clin Immunol. 2014;134(3):645–652. doi: 10.1016/j.jaci.2014.04.039.[PMC free article] [PubMed] [Cross Ref]
Sato S, Tachimoto H, Shukuya A, Kurosaka N, Yanagida N, Utsunomiya T, Iguchi M, Komata T, Imai T, Tomikawa M, et al. Basophil activation marker CD203c is useful in the diagnosis of hen’s egg and cow’s milk allergies in children. Int Arch Allergy Immunol. 2010;152(Suppl 1):54–61. doi: 10.1159/000312126. [PubMed] [Cross Ref]
Tokuda R, Nagao M, Hiraguchi Y, Hosoki K, Matsuda T, Kouno K, Morita E, Fujisawa T. Antigen-induced expression of CD203c on basophils predicts IgE-mediated wheat allergy. Allergol Int. 2009;58(2):193–199. doi: 10.2332/allergolint.08-OA-0023. [PubMed] [Cross Ref]

