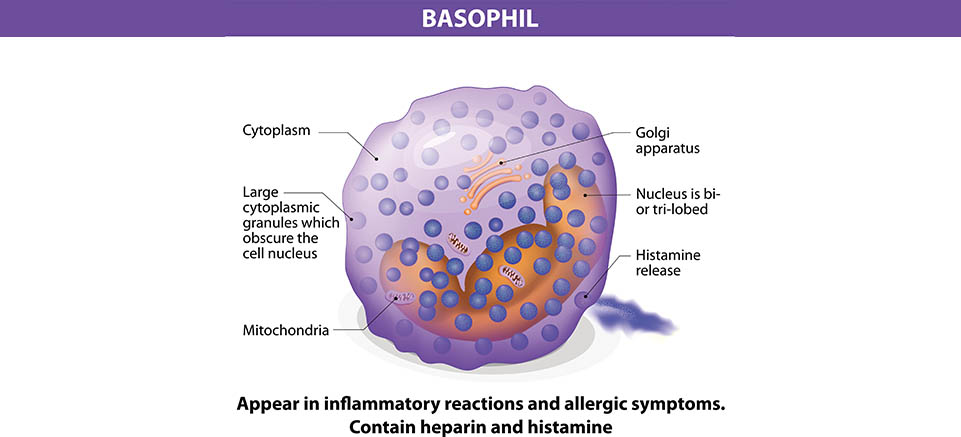
Allergies are one of the fastest growing maladies affecting first world countries. In fact, before 1980 peanut allergies, now so commonly and dangerously found in children, were virtually unheard of in both medical journals and the media. In the USA alone, the CDC reports that between 1997 and 2008, food allergies in children have risen […]