Fc Receptors (FcR) and their Relevance to Flow Cytometry
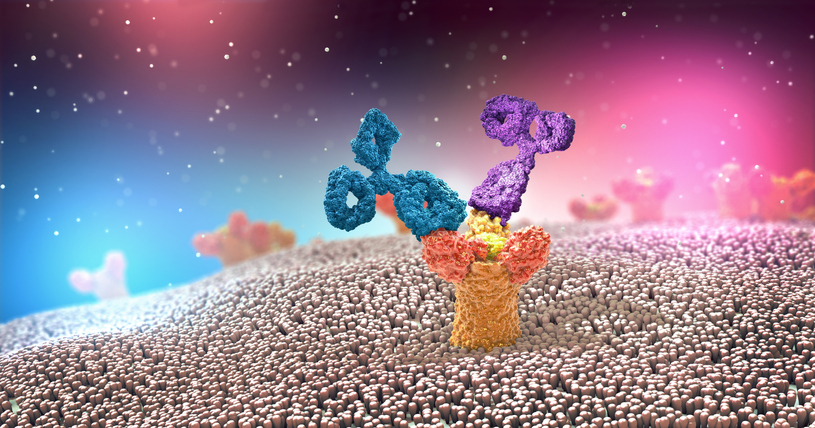
Antibodies, also known as Immunoglobulins or Igs, consist of two heavy and two light chains. The antigen binding part of the antibody (shown in purple, Figure 1), is the variable region and capable of recognizing a specific antigen out of millions of possible antigens. The remaining sequence of the antibody is relatively conserved, and termed the constant region (shown in turquoise, Figure 1). As diagramed below, the antibody consists of two main structures, the Fab domain and the Fc domain. There are several subclasses of antibodies including IgA, IgE, IgM, and IgG1, IgG2, IgG3 and IgG4. Antibodies play two main roles: to recognize pathogens (on the antigen binding end), and to initiate effector function to clear them out (though the Fc portion). The Fc portion of antibodies is recognized by Fc Receptors (FcR), on immune cells. FcR are found primarily on cells of the myeloid lineage (monocytes, neutrophils, dendritic cells DC, mast cells, and eosinophils), and in a more restricted manner, on NK cells and B cells. In this way, FcR link the adaptive immune and innate immune systems. When FcR recognize the aggregated Fc-domains on antibody coated cells or immune complexes, effector functions, including phagocytosis by monocytes and macrophages, antibody-dependent cell–mediated cytotoxicity (ADCC) by NK cells, neutrophil degranulation and platelet activation, are triggered (1). Because Fc domains are conserved between antibodies of a given subclass, FcRs regulate antibody-induced immune responses irrespective of antigen specificity (2).

Figure 1. Antibody Structure
FcR are named by the type of antibody that they recognize. FcR that bind the most common class of antibody, IgG, are called Fc-gamma receptors (FcγR), those that bind IgA are called Fc-alpha receptors (FcαR) and those that bind IgE are called Fc-epsilon receptors (FcεR). FcR expression is primarily found on cells of the innate immune system (neutrophils, monocytes, macrophages, dendritic cells) NK cells (FcγRIIa and FcγRIIc) mast cells (FcϵRI) and, in restricted manner, B cells (FcγRIIb). In humans, activating FcγR’s are FcγRI (CD64, high affinity), FcγRIIa (CD32a), FcγRIIc (CD32c), and FcγRIIIa (CD16a), and FcγRIIIb (CD16b). FcγRIIb is the single inhibitory receptor. The intricacies of the various FcR are summarized in Table 1.
Table 1. FcR Expression on Immune cells, their ligands and affinity

*FcγRIIB is an inhibitory receptor.
Antigen presenting cells (macrophages and DC) express both activating and the inhibitory FcγR (Figure 2). FcR transmit their signals to the immune cell via immunoreceptor tyrosine-based activation motifs (ITAM), and immunoreceptor tyrosine-based inhibitory motifs (ITIM), respectively (2,3). FcγRIIIb lacks intracellular signaling and ITAM motifs, but it is still considered an activating receptor (2).

Figure 2. Taken from Teige et al., 2019, diagram of activating and inhibitory FcγR on the surface of a macrophage cell.
FcR biology is elegant and complex. The receptors are diverse and FcR (in all their forms) provide more than a simple “pathogen disposal mechanism” (4). FcR play multiple roles in desirable, and undesirable, immunomodulation including:
- Humoral immune response against infection
- Regulation of B cell function
- Delivery of antibody captured antigen to Antigen Presenting Cells (APC)
- Control of transport and catabolism of circulating IgG
- Immune Complex Clearance
- IgE-dependent Allergy response
- Pathogenesis of Heparin-Induced Thrombocytopenia
Flow cytometry uses fluorescently tagged antibodies as probes to recognize markers on the cell surface, and intracellular markers within cells of interest. Non-specific binding of antibodies to FcR can occur through the Fc portion, resulting in increased false positive staining and meaningless data. Most antibodies to human antigens are raised in mice.
A study by Anderson et al., 2016 found the highest non-specific binding of mouse antibodies was to human peripheral blood mononuclear cells (MNCs) and monocyte-derived macrophages (MDMs). Mouse IgG1 and IgG2a, but not IgG2b, showed high nonspecific binding to monocytes and MDMs. Human B cells, T cell and NK cells did not bind the mouse isotypes evaluated (IgG1, IgG2a and IgG2b). Notably, isotype controls, widely used in flow cytometry as gating controls, can also bind non-specifically to FcR through their Fc, and thus can be unreliable as controls. Therefore blocking of FcR is highly recommended (5).
If cells of interest highly express Fc-receptors on their surface (in particular monocytes, macrophages and B cells) or they have been cultured in serum free medium, it is advisable to block nonspecific binding (of monoclonal antibodies) by pre-incubation of cells with a Fc Block (for staining of whole blood this is not necessary, because serum in high concentration is present during staining). Various commercial products exist to block non-specific Fc binding including the use of human serum, purified human IgG, recombinant human Fc proteins, and anti-CD16 (FcγRIII) and CD32 (FcγRII) that cross react to the species used (in this case human). It must be confirmed that these FcR block antibodies do not interfere with a target of interest (for example CD16). Another way to block FcR is to use normal serum from the species in which the primary antibody was raised (i.e., if a mouse anti-Human antibody is used, use 5% normal mouse serum to block).
FcR are very important to the biotech industry because therapeutic antibodies (engineered Human IgGs), and variations of whole antibodies, like Fc-Fusion proteins as are developed as drugs. Therapeutic antibodies can be intentionally designed to reduce binding to FcR (i.e., IgG4 and aglycosylated IgG1), or be designed to increase binding to FcR in order to enhance effector function (fucosylation) (6). Retrospective studies have shown that patients with allelic variations of activating FcγR (FcγRIIIa and FcγRIIa) have improved responses to the cancer targeting antibodies Rituximab, Herceptin and Cetuximab (2). Finally, FcR themselves are candidates for drug targets; for the treatment of autoimmune and inflammatory conditions (1), and FcγRIIb (the inhibitory FcR), for cancer (2).
FCSL is a contract flow cytometry lab with expertise in complex flow cytometry panel & assay design, including Receptor Occupancy Assays (ROA), Immunophenotyping, Cell Signaling, Cell Cycle Analysis, and Cell Functional Assays. We routinely run flow cytometry assays in support of basic research, clinical and non-clinical studies and are experts in the validation of flow cytometry assays. We believe that flow cytometry can be used to answer any question and solve any problem in the drug development process. If your research requires the power of flow cytometry, contact us for more information.
References:
- Bosques CJ, Manning AM. Fc-gamma receptors: Attractive targets for autoimmune drug discovery searching for intelligent therapeutic designs. Autoimmun. Rev. 2016;15:1081–1088.
- Teige I, Mårtensson L, Frendéus BL. Targeting the antibody checkpoints to enhance cancer immunotherapy-focus on FcγRIIb. Front. Immunol. 2019;10:1–14.
- Koenderman L. Inside-out control of Fc-receptors. Front. Immunol. 2019;10.
- Hogarth PM. Fc Receptors: Introduction. Immunol. Rev. 2015;268:1–5.
- Andersen MN, Al-Karradi SNH, Kragstrup TW, Hokland M. Elimination of Erroneous Results in Flow Cytometry Caused by Antibody Binding to Fc Receptors on Human Monocytes and Macrophages.
- Wang X, Mathieu M, Brezski RJ. REVIEW IgG Fc engineering to modulate antibody effector functions. Protein Cell 9. Available at: http://www.imgt.

